Meiotic recombination is a critical step in gametogenesis for many organisms, enabling the creation of genetically diverse haploid gametes. In each meiotic cell, recombination is initiated by numerous DNA double-strand breaks (DSBs) created by Spo11, the evolutionarily conserved topoisomerase-like protein, but how these DSBs are distributed relatively uniformly across the four chromatids that make up each chromosome pair is poorly understood.
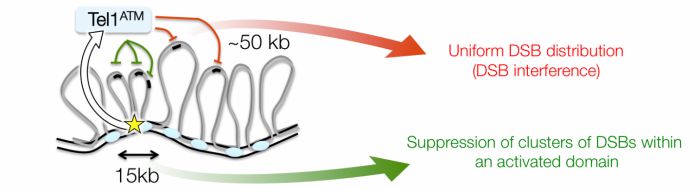
In this study, we employed Saccharomyces cerevisiae to demonstrate for the first time distance-dependent DSB interference in cis (in which the occurrence of a DSB suppresses adjacent DSB formation)—a process that we find is mediated by the conserved DNA damage response kinase, Tel1 (the budding yeast orthologue of the human tumour suppressor gene, ATM). The inhibitory function of Tel1 acts on a relatively local scale, while over large distances DSBs have a tendency to form independently of one another even in the presence of Tel1. Notably, over very short distances, loss of Tel1 activity causes DSBs to form concertedly within individual cells, within discrete 15-20kb long regions that correlate with the inferred position of chromatin loops.
Our observations support a hierarchical view of recombination initiation where Tel1ATM prevents clusters of DSBs forming within these loop domains, and further suppresses DSBs within the surrounding chromosomal region. The concept that DSBs might cluster in individual cells is unprecedented, and will have a major impact on the way researchers consider and model rates and distributions of recombination occurring during gametogenesis. Overall, the collective negative regulation on DSB formation mediated by Tel1ATM will help to ensure that recombination events are dispersed evenly and arranged optimally for genetic exchange and efficient chromosome segregation.
Our work helps to complete the picture initiated by ourselves and others concerning the complex role that ATM and ATR have on meiotic DSB regulation (reviewed by our team in Cooper et al, Experimental Cell Research 2014 and in Cooper et al, Cell Cycle 2016).