Garcia et al. 2011 Nature 144(5): 719-7
In order to be efficiently repaired, the ends of DNA breaks must be recognised and processed appropriately for either of two general repair pathways: Non-homologous end-joining (NHEJ) which directly ligates (sticks) the two sides of the broken DNA molecule back together, or Homologous recombination (HR), which involves degradation of one strand of the DNA molecule on both sides of the DNA break to create long regions of single-stranded DNA (ssDNA). The ssDNA regions are used to search for and pair up with identical DNA sequences present elsewhere in the genome (usually the sister chromatid or the homologous chromosome), and initiate a high-fidelity templated DNA repair reaction.
In meiotic cells, it is these damage-induced HR DNA repair reactions that create the transient links between homologous chromosomes that are essential for reductional chromosome segregation. Furthermore, it is a consequence of the HR DNA repair reaction that causes the genetic reassortments that are the signature of meiotic cells: i.e. all products of meiotic cells (eggs and sperm) are slightly different from one another, being a unique amalgamation of the parental (mother and father's) genetic information.
One of the key enzymatic components of DSB repair is the multifunctional Mre11-Rad50-Nbs1 complex. The Mre11 enzyme binds DNA ends and displays both endonucleolytic and exonucleolytic nuclease activity (it can cut DNA molecules both internally - "Endo", and externally from an end - "Exo"); however, a precise explanation for the role of the Mre11 exonuclease activity has been lacking.
In the recent study by Valerie Garcia, we report that DSB ends in meiosis are coordinately processed by the complementary nuclease activities of the bifunctional Mre11 enzyme, along with a second factor, Exo1. We propose that this reaction occurs in three phases:
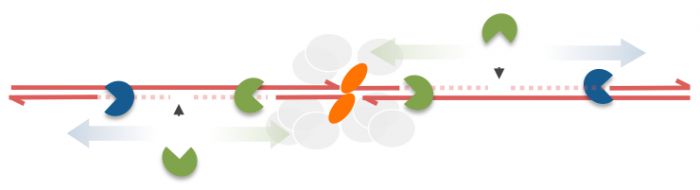
1/ First, single-stranded nicks are made in 5'-ending strands (the strands that Spo11 is covalently attached to) on both sides of the DSB end. These nicks create intermediates called Spo11-oligonucleotides that can be detected in whole cell extracts by direct end-labelling (Neale et al 2005). We now propose that these nicks originate more distally from the Spo11-DSB ends than first thought - as far as 300 DNA base-pairs away. We propose that these nicks create a starting point for the complementary exonuclease activities of Mre11 and Exo1 (step 2 and 3) to create the single-stranded DNA necessary for HR.
2/ In the second phase, we propose that the 3'-5' exonuclease activity of Mre11 progressively shortens the Spo11-oligonucleotide DNA molecules, finally ending up with the relatively short (10-40 nt fragments) observed in wild-type cells. Indeed, based on their protection from degradation by exogenous nucleases, we propose that the final length of the Spo11-oligos refers to the amount of DNA covered (protected) by the proteins involved in Spo11-DSB formation. Essentially, the final Spo11-oligonulceotide size may be the "footprint" of the Spo11-DSB-forming machine.
3/ The third step involves resection away from the DSB ends by the highly processive 5'-3' exonuclease Exo1. It is thus a combination of Mre11 and Exo1 that create the large amounts of single-stranded DNA that are used to initiate the strand pairing and DNA repair reactions. Mre11 creates the 50-300 nt closest to the DSB end, Exo1 creates 800-1000 nt outside of this central area.
Our work is significant for a number of reasons: Primarily, it provides one of the first explanations and descriptions for the evolutionarily conserved Mre11 3'-5' exonuclease activity; The study further provides detail into the highly conserved and general processes of DSB repair; Finally, the study suggests new mechanistic details about the poorly understood mechanism of meiotic recombination initiation (e.g. the footprint of the Spo11-enzyme machine).