Some of our recently published structures
|
|
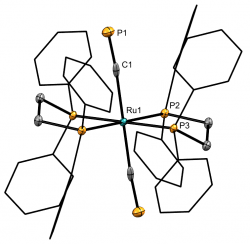
[Ru(dppe)2(C≡P)2]Chem. Eur. J., 2024, e202303370 We recently reported the first example of a monometallic bis(cyaphido) complex. Considered alongside our previously reported trans cyaphido-carbonyl, and a novel trans-cyano-cyaphide complex (see below), structural and computational data substantiated previous assertion of the enhanced π-acceptor character of cyaphide over cyanide, while also revealing for the first time an appreciable π-donor component. The work was highlighted in Chemistry Views Magazine. |
|
|
[Ru(dppe)2(C≡N)(C≡P)]Chem. Eur. J., 2021, 27, 16242 - 16246 Alongside our bis(cyaphido) complex we reported this novel trans-cyano-cyaphido complex, the first time cyanide and cyaphide have featured as ligands at the same metal centre. Computational study of this complex, alongside the cyaphide and carbonyl (see above) allowed an unprecedented insight into the relative donor/acceptor character of this series of ligand analogues. This work was highlighted in Chemistry Views Magazine. |
|
|
[Mo(CO)3{η5-(C6H4P2BPh)}{η1-Mo(CO)3(TMEDA)}2].[μ-Li(THF)].[μ-Li(TMEDA)]Chem. Eur. J., 2021, 27, 16242 - 16246 We recently reported a simple synthetic route to the first example of a diphosphaborolediide - a missing member of the 'hetero-Cp' family of ligands. The aromatic dianion is obtained as its dilithium salt as either a monomeric tmeda solvate, or an oligomeric thf-solvated complex. The ligand also coordinates as a pi-ligand to molybdenum(0), demonstrating its suitability for low- and zero-valent metal centres. This work was highlighted in Chemistry Views Magazine. |
|
|
[C6H4P2BPh].2[Li(THF)n] A benzodiphosphaborolediideChem. Eur. J., 2021, 27, 16242 - 16246 We recently reported a simple synthetic route to the first example of a diphosphaborolediide - a missing member of the 'hetero-Cp' family of ligands. The aromatic dianion is obtained as its dilithium salt as either a monomeric tmeda solvate, or an oligomeric thf-solvated complex. The ligand also coordinates as a pi-ligand to molybdenum(0), demonstrating its suitability for low- and zero-valent metal centres. This work was highlighted in Chemistry Views Magazine. |
|
|
[C6H4P2BPh].2[Li(TMEDA)] A benzodiphosphaborolediideChem. Eur. J., 2021, 27, 16242 - 16246 We recently reported a simple synthetic route to the first example of a diphosphaborolediide - a missing member of the 'hetero-Cp' family of ligands. The aromatic dianion is obtained as its dilithium salt as either a monomeric tmeda solvate, or an oligomeric thf-solvated complex. The ligand also coordinates as a pi-ligand to molybdenum(0), demonstrating its suitability for low- and zero-valent metal centres. This work was highlighted in Chemistry Views Magazine. |
- Archive 2020
-

m-{-C(O)-NC5H3-(C(O)PMe)}2
J. Org. Chem., 2020 85, 14697-14707
We recently elaborated our range of diphosphametacyclophanes to include a series of macrocycles featuring substitution in the aromatic 5-position, along with analogues based on pyridyl units and a P-phenyl analogue of our original metacyclophane. Spectroscopic, cyclic voltammetry and DFT studies probled the photophysical properteis of these molecules, demonstrating their similarity to precedent diketophosphanyl derivatives explored for opto-electronic applications.

m-{-C(O)-C6H4-(C(O)PPh)}2
J. Org. Chem., 2020 85, 14697-14707
We recently elaborated our range of diphosphametacyclophanes to include a series of macrocycles featuring substitution in the aromatic 5-position, along with analogues based on pyridyl units and a P-phenyl analogue of our original metacyclophane. Spectroscopic, cyclic voltammetry and DFT studies probled the photophysical properteis of these molecules, demonstrating their similarity to precedent diketophosphanyl derivatives explored for opto-electronic applications.

m-{-C(O)-C6H3(p-C6H4CN)-(C(O)PMe)}2
J. Org. Chem., 2020 85, 14697-14707
We recently elaborated our range of diphosphametacyclophanes to include a series of macrocycles featuring substitution in the aromatic 5-position, along with analogues based on pyridyl units and a P-phenyl analogue of our original metacyclophane. Spectroscopic, cyclic voltammetry and DFT studies probled the photophysical properteis of these molecules, demonstrating their similarity to precedent diketophosphanyl derivatives explored for opto-electronic applications.

m-{-C(O)-C6H3tBu-(C(O)PMe)}2
J. Org. Chem., 2020 85, 14697-14707
We recently elaborated our range of diphosphametacyclophanes to include a series of macrocycles featuring substitution in the aromatic 5-position, along with analogues based on pyridyl units and a P-phenyl analogue of our original metacyclophane. Spectroscopic, cyclic voltammetry and DFT studies probled the photophysical properteis of these molecules, demonstrating their similarity to precedent diketophosphanyl derivatives explored for opto-electronic applications.

m-{-C(O)-C6H3Ph-(C(O)PMe)}2
J. Org. Chem., 2020 85, 14697-14707
We recently elaborated our range of diphosphametacyclophanes to include a series of macrocycles featuring substitution in the aromatic 5-position, along with analogues based on pyridyl units and a P-phenyl analogue of our original metacyclophane. Spectroscopic, cyclic voltammetry and DFT studies probled the photophysical properteis of these molecules, demonstrating their similarity to precedent diketophosphanyl derivatives explored for opto-electronic applications.

m-{-C(O)-C6H3Me-(C(O)PMe)}2
J. Org. Chem., 2020 85, 14697-14707
We recently elaborated our range of diphosphametacyclophanes to include a series of macrocycles featuring substitution in the aromatic 5-position, along with analogues based on pyridyl units and a P-phenyl analogue of our original metacyclophane. Spectroscopic, cyclic voltammetry and DFT studies probled the photophysical properteis of these molecules, demonstrating their similarity to precedent diketophosphanyl derivatives explored for opto-electronic applications.

m-{-C(O)-C6H3I-(C(O)PMe)}2
J. Org. Chem., 2020 85, 14697-14707
We recently elaborated our range of diphosphametacyclophanes to include a series of macrocycles featuring substitution in the aromatic 5-position, along with analogues based on pyridyl units and a P-phenyl analogue of our original metacyclophane. Spectroscopic, cyclic voltammetry and DFT studies probled the photophysical properteis of these molecules, demonstrating their similarity to precedent diketophosphanyl derivatives explored for opto-electronic applications.

[W(CO)5(tBu)P{C(O)(CH2)3C(O)}]
Dalton Trans., 2020 49, 5482-5492
We recently reported the first examples of phosphinane-2,6-diones, with a range of P-substituents. These hetercyclic diketyls exhibit a relatively stabilised lone-pair that appears resistant to oxidation and is a somemwhat weaker σ-donor than typical phosphines, which they also lie below in the trans-influence series.

[W(CO)5(Me)P{C(O)(CH2)3C(O)}]
Dalton Trans., 2020 49, 5482-5492
We recently reported the first examples of phosphinane-2,6-diones, with a range of P-substituents. These hetercyclic diketyls exhibit a relatively stabilised lone-pair that appears resistant to oxidation and is a somemwhat weaker σ-donor than typical phosphines, which they also lie below in the trans-influence series.

[W(CO)5(Ph)P{C(O)(CH2)3C(O)}]
Dalton Trans., 2020 49, 5482-5492
We recently reported the first examples of phosphinane-2,6-diones, with a range of P-substituents. These hetercyclic diketyls exhibit a relatively stabilised lone-pair that appears resistant to oxidation and is a somemwhat weaker σ-donor than typical phosphines, which they also lie below in the trans-influence series.

cis-[PtCl2(Ph)P{C(O)(CH2)3C(O)}PEt3]
Dalton Trans., 2020 49, 5482-5492
We recently reported the first examples of phosphinane-2,6-diones, with a range of P-substituents. These hetercyclic diketyls exhibit a relatively stabilised lone-pair that appears resistant to oxidation and is a somemwhat weaker σ-donor than typical phosphines, which they also lie below in the trans-influence series.

1-Mesitylphosphinane-2,6-dione
Dalton Trans., 2020 49, 5482-5492
We recently reported the first examples of phosphinane-2,6-diones, with a range of P-substituents. These hetercyclic diketyls exhibit a relatively stabilised lone-pair that appears resistant to oxidation and is a somemwhat weaker σ-donor than typical phosphines, which they also lie below in the trans-influence series.

1-Phenylphosphinane-2,6-dione
Dalton Trans., 2020 49, 5482-5492
We recently reported the first examples of phosphinane-2,6-diones, with a range of P-substituents. These hetercyclic diketyls exhibit a relatively stabilised lone-pair that appears resistant to oxidation and is a somemwhat weaker σ-donor than typical phosphines, which they also lie below in the trans-influence series.
- Archive 2018-19
-

[Ru(dppe)2(C≡P)]+
Inorg. Chem., 2019 58, 14800-14807
In a particularly exciting step forward for this field, we have now achieved access - by halide abstraction from the trans-halide complexes - to a coordinately unsaturated ruthenium complex of the cyaphide ligand, enabling us to introduce trans-ligands to order (e.g. the carbonyl below). This circumvents the difficulties associated with installing the cyaphide ligand as a final step, which have previously impeded much work in this area.

[Ru(dppe)2(CO)(C≡P)]+

[Ru(dppe)2Br(C≡P)]
The trans-halo complexes [Ru(dppe)2X(CP)] (X = Cl, Br, I) are easily obtained by methide exchange from the parent trans-methyl complex (below) with ZnX2 / PPh3. This represents the first example of controlled reativity int he coordination sphere of a cyaphide complex - previous such complexes have been found to either be inert, or prone to decomposition with loss of cyaphide.
[Ru(dppe)2Me(C≡P)]
Inorg. Chem., 2019 58, 14800-14807
The first trans-alkyl-cyaphide complex was prepared using established methods for cyaphide synthesis, commencing from the methyl cation [Ru(dppe)2Me]+, obtained by TlOTf methide metathesis from the dimethyl complex [Ru(dppe)2Me2].
![Molecular structure of [Ru(dppe)2(CCCO2Me)(CP)]](/wcm/assets/media/88/content/59707.250x173.jpg)
[Ru(dppe)2(C≡CCO2Me)(C≡P)]
Dalton Trans., 2019 48, 8131-8143
As part of an extended study into the synthesis and electronic nature of complexes featuring the cyaphide ligand through-conjugated (via ruthenium) to a trans-alkynyl we published a range of structures (below), including this structure of one of the first cyaphide complexes we reported (Dalton Trans., 2014 43, 9004 - 9007).
![Molecular structure of [Ru(dppe)2(CCC6H4F)(CP)]](/wcm/assets/media/88/content/59706.250x176.jpg)
[Ru(dppe)2(C≡CC6H4F)(C≡P)]
![Molecular structure of [Ru(dppe)2(CCC6H4CO2Me)(CP)]](/wcm/assets/media/88/content/59703.250x174.jpg)
[Ru(dppe)2(C≡CC6H4CO2Me)(C≡P)]
![Molecular structure of [Ru(dppe)2(CCC6H4F)(PCSiMe3)]+](/wcm/assets/media/88/content/59705.250x129.jpg)
[Ru(dppe)2(η1-P≡CSiMe3)(C≡CC6H4F)]+
Dalton Trans., 2019 48, 8131-8143
As we previously described, each of the cyaphide complexes is prepared by base-induced desilylation of the η1-bound phosphaalkyne P≡CSiMe3, within the coordination sphere of the respective ruthenium alkynyl complex.
![Molecular structure of [Ru(dppe)2(CCC6H4Me)(PCSiMe3)]+](/wcm/assets/media/88/content/59704.250x160.jpg)
[Ru(dppe)2(η1-P≡CSiMe3)(C≡CC6H4Me)]+
Dalton Trans., 2019 48, 8131-8143
[{Ru(dppe)2}2{μ-(C≡C)2C6H4-p}(C≡P)2]
Dalton Trans..2018, 47, 4428 - 4432
We have recently reported the first compound to incorporate two, through-conjugated 'C≡P' moieties; thi unprecedented bimetallic bis-cyaphide complex.
[{Ru(dppe)2}2{μ-(C≡C)2C6H4-p}(η1-P≡CSiMe3)2]
Dalton Trans..2018, 47, 4428 - 4432
The first bimetallic complex to feature two η1-phosphaalkyne ligands as part of an extended structure. This compound serves as precursor in our recent report of the first complex to feature two 'C≡P' ligands as part of a single through-conjugated chain. - Archive 2016-17
-
![Molecular structure of [Ru{P(H)ClCH2(SiMe3)}Cl2(CO)(PPh3)2]](/wcm/assets/media/88/content/45249.250x254.jpg)
[Ru{η1-P(H)Cl–CH2SiMe3)}Cl2(CO)(PPh3)2]
Organometallics.2017, 36, 435 - 442
We recently discovered a remarkably facile cascade reaction, resulting in the double hydrochlorination of our phosphaalkenyl complexes to yield rare examples of the alkylhydrohalophosphane ligand, stabilised in the metal coordination sphere. The conformational arrangement observed here in the solid state is apparently retained in solution, as evidenced by a Karplus-like coupling dependence for the H-P-Ru-P interactions.![Molecular structure of [Ru{eta-1;eta-2-P(pz*)=C(SiMe2C6H4CF3)H}(CO)(PPh3)2]](/wcm/assets/media/88/content/43192.250x199.jpg)
[Ru{η1-N:η2-P,C-P(pz*)=CH(SiMe2C6H4CF3)}(CO)(PPh3)2]
Inorganics. 2016, 4, 30
We recently extended our work on chelated pyrazolylphosphaalkene complexes of ruthenium, to consider the structural and spectroscopic features of range os such compounds. This included only the second crystallographically characetised example.
O=P(o-Tol)3
Eur. J. Inorg. Chem. 2016, 4076 - 4082
- Archive 2015
-
![x-ray struture of [Ru{P=C(SiMe2Tol)H}Cl(CO)(PPh3)2]](/wcm/assets/media/88/content/29768.250x219.jpg)
[Ru{P=C(SiMe2Tol)H}Cl(CO)(PPh3)2]
Organometallics 2015, 34, 2533 - 2542
We recently reported the first structural data for 5-coordinate ruthenaphosphaalkenyl complexes.![x-ray struture of [Ru{P=C(SiMe2Ph)H}Cl(CO)(PPh3)2]](/wcm/assets/media/88/content/29767.250x257.jpg)
[Ru{P=C(SiMe2Ph)H}Cl(CO)(PPh3)2]
We recently reported the first structural data for 5-coordinate ruthenaphosphaalkenyl complexes.![x-ray struture of [Ru{P=C(SiMe3)H}Cl(CO)(PPh3)2]](/wcm/assets/media/88/content/29766.250x258.jpg)
[Ru{P=C(SiMe3)H}Cl(CO)(PPh3)2]
We recently reported the first structural data for 5-coordinate ruthenaphosphaalkenyl complexes. - Archive 2014
-

[Rh(CO)Cl{PFu2(C2H4B(C8H14)}2]
Eur. J. Inorg. Chem. 2014, 5053 - 5062

[Cp*IrCl2(PPh2nPr)]

Ph2BC(Ph)=C(Ph)PPh2

Bu2BC(Bu)=C(Ph)PPh2

[Ru(dppe)2(C≡CC6H4OMe)(C≡P)]
Dalton Trans., 2014 43, 9004 - 9007
We recently described the first examples of cyaphide-alkynyl complexes, which show through-conjugation of the π-systems.

[Ru(dppe)2(η1-P≡CSiMe3)(C≡CCO2Me)]+
Dalton Trans., 2014 43, 9004 - 9007
An η1-complex of the phosphaalkyne Me3SiC≡P

[Ru(dppe)2(C≡CCO2Me)Cl]
Dalton Trans., 2014 43, 9004 - 9007
- Archive 2012-13
-

[Ru{η1-N:η2-P,C-P(pz)=CH(SiMe3)}(CO)(PPh3)2]
Organometallics 2013, 32, 2501-2504
We recently described the formation of unprecedented η1:η2-chelating pyrazolylphosphaalkene complexes of ruthenium(0), via nucelophilic attack of pyrazolate at ruthenium(II) phosphaalkenyl complexes.

[Ru{η1-P(HgPh)=CH(SiMe3)}Cl2(CO)(PPh3)2]
Organometallics 2013, 32, 2501-2504
An η1-mercuriophosphaalkene complex of ruthenium(II), obtained by reaction of a ruthenium(II) phosphaalkenyl complex with PhHgCl.

m-{-C(O)-C6H4(C(O)PMe)}2
Chem. Commun. 2012, 48, 5766-5768
We have prepared, via a mild protocol, the first example of a diphosphametacyclophane.

Me3SiCH2P{P(SiMe3)2}2
Dalton Trans. 2012, 41, 278-284
A very reactive polysilyltriphosphane, which behaves as a bidentate chelating ligand.


![X-ray strucutre of trans-[Ru(dppe)2CN(CP)]](/wcm/assets/media/88/content/79687.250x307.png)


